 Hello good people of Earth and...everywhere else! I'm back again, and today we are going to review atomic structure, and learn about something new that we call chemical bonds.
Hello good people of Earth and...everywhere else! I'm back again, and today we are going to review atomic structure, and learn about something new that we call chemical bonds. Atoms are the first step to learning about chemical bonds. The atom's nucleus is located in the center, and it is surrounded by a cluster of protons (positively charged), and neutrons (no charge). Around all of this are electron shells, which hold a certain number of electrons inside each one. On the first shell, there are two electrons, on the second there are eight, and on the last there are sixteen. All of these electrons move around the nucleus in a roughly spherical orbit inside of a cloud. When the outermost shell of an atom is completely full, the atom is stable, meaning it will not try to bond with another atom. When the shell is unstable, however, the atom will seek out other unstable atoms, and try to bond with them.
Atoms are the first step to learning about chemical bonds. The atom's nucleus is located in the center, and it is surrounded by a cluster of protons (positively charged), and neutrons (no charge). Around all of this are electron shells, which hold a certain number of electrons inside each one. On the first shell, there are two electrons, on the second there are eight, and on the last there are sixteen. All of these electrons move around the nucleus in a roughly spherical orbit inside of a cloud. When the outermost shell of an atom is completely full, the atom is stable, meaning it will not try to bond with another atom. When the shell is unstable, however, the atom will seek out other unstable atoms, and try to bond with them. Now let's move on to types of bonds, shall we? The first type of bond I will talk about covalent bonds. A covalent bond is the sharing of electrons between two unstable atoms, a great example of this being oxygen, which is vital to our existence. The next kind is an ionic bond, which happens when one atom takes an electron from another atom("Talk about rude!"). One of the atoms becomes negative, and the other becomes positive, and this process occurs through electrostatic attraction. Electrostatic attraction is when two atoms with opposite charges bond("Opposites attract!"). Sodium chloride is a great example of an electrostatic attraction.
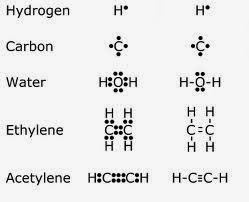
Now let's move on to types of bonds, shall we? The first type of bond I will talk about covalent bonds. A covalent bond is the sharing of electrons between two unstable atoms, a great example of this being oxygen, which is vital to our existence. The next kind is an ionic bond, which happens when one atom takes an electron from another atom("Talk about rude!"). One of the atoms becomes negative, and the other becomes positive, and this process occurs through electrostatic attraction. Electrostatic attraction is when two atoms with opposite charges bond("Opposites attract!"). Sodium chloride is a great example of an electrostatic attraction.
Hope you all enjoyed this! I'll be back with another as soon as possible, but until then, please leave a comments, I'd love to hear what you think! Au revoir, mes amis!

Absolutely per-fect *Lee-Roy 'z' snap* just check over some things, but overall 99%!
ReplyDeleteGreat job! A+
ReplyDeleteGreat blog and had lots of good pics. Grade- A
ReplyDeleteNice pics and great info. would not change anything.
ReplyDeleteGood layouts and I like your multimedia. But it would be little better if you had more infromation about Covalent and Ionic bonds!
ReplyDeleteA+